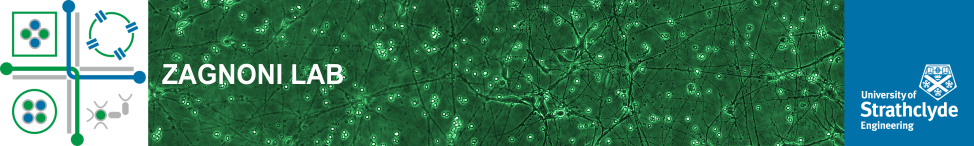
The aim of this project is to investigate the characteristics of emulsion-based techniques for the formation and long term culture of multicellular glioma cancer spheroids and apply these findings to assess the cytotoxic effect of X-ray radiation on spheroids. The microfluidics will allow the identification of the effects produced on the proliferative state of the spheroids due to the compartmentalised nature of the emulsions, creating a model that mimics tumour growth and tumour quiescence.
 .
.
Dr Kay McMillan
Co-Investigators:
Dr M. Zagnoni, Univ. Strathclyde
Dr M. Boyd, Univ. Strathclyde
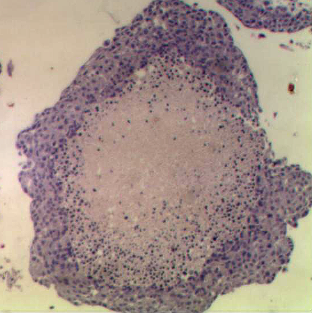
The aim of this project is to develop a miniaturised technology to screen liver toxicity in vitro, using 3D hepatocyte structures. This is done using emulsion techniques that enable the long-term culture of primary liver cells to be obtained in 3D. Microfluidic procedures will be developed to create an in vitro model of liver tissue that mimics in vivo hepatic conditions and is able to predict formation of reactive intermediates from foreign compounds, e.g. candidate pharmaceuticals, which have the potential to cause toxicity in vivo.
 .
.
Myndert Claasen
Co-Investigators:
Dr M. Zagnoni, Univ. Strathclyde
Prof H. Grant, Univ. Strathclyde
Dr D. Watson, Univ. Strathclyde
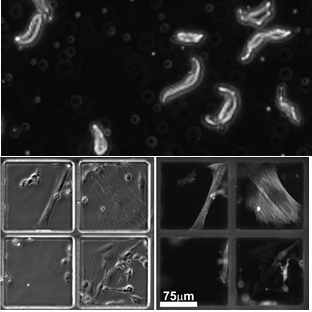
A microfluidic approach to investigating vascular cell
fate at the single-cell level
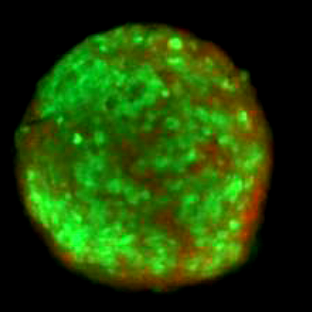
Cardiovascular disease remains the leading cause of death worldwide and stems from changes in blood vessel structure. As a result of cell accumulation and growth in the vessel’s inner layers, “plaques” form that narrow arteries and obstruct blood flow. However, uncertainties remain over the origin of the cells populating plaques, the behavioural changes they undergo during disease and the role of interactions between different cell types. This project will shed new light on the mechanisms causing abnormal proliferation of vascular cells and the cell sub-populations involved. Understanding this, and especially the variations existing at the single cell level, is critical to developing new treatments. Therefore, we will develop a new methodology to continuously track the functional changes that single cells from the vascular wall undergo in response to various stimuli and drug treatments.
 .
.
Ellis Smith
Co-Investigators:
Dr M. Sandison, Univ. Strathclyde
Dr M. Zagnoni, Univ. Strathclyde
AMS Biotechnology (Europe) Ltd

© Copyright 2017-2021 by Michele Zagnoni